“Hydrochloric Acid, 37% ACS Reagent, GL 2.5 Liter, case/6” has been added to your cart. View cart
Carbon Disulfide 99.9%+, ACS Reagent, 2 Liter
$226.03
- Part Number: MIL-180173-2L
- Lead Time: 2-4 weeks
- Quantity: each
- Size: 2 liter / 64 ounce
- Style: chemical
Category: ACS Grade Chemicals
Description
Carbon Disulfide >/=99.9% ACS Reagent & 2 Liter
- CAS Number 75-15-0
- Empirical Formula (Hill Notation) CS2
- Molecular Weight 76.14
- Beilstein/REAXYS Number 1098293
- EC Number 200-843-6
- MDL number MFCD00011321
- PubChem Substance ID 329751607
| Grade | ACS reagent |
| Vapor Density | 2.67 (vs air) |
| Vapor Pressure | 5.83 psi |
| InChI Key | QGJOPFRUJISHPQ-UHFFFAOYSA-N |
| Assay | ≥99.9% |
| Autoignition Temperature | 212°F |
| expl. lim. | 50 % |
| Impurities | H2S, passes test (lim. ~1.5 ppm)
SO2, passes test (lim ~2.5 ppm) ≤0.05% water |
| evapn. residue | ≤0.002% |
| Color | APHA: ≤10 |
| Refractive Index | n20/D 1.627 (lit.) |
| bp | 46°C (lit.) |
| mp | −112-−111°C (lit.) |
| Density | 1.266 g/mL at 25°C (lit.) |
General description
Carbon disulfide (CS2) is a colorless liquid. It is present in atmosphere and natural waters in the form of sulfur gas. It is responsible for the production of sulfur dioxide (SO2) in the atmosphere (stratosphere and/or troposphere). Its lifetime in the atmosphere has been proposed to be around 12 days. It is a non-polar solvent.
Carbon disulfide is a highly volatile, flammable liquid with low ignition temperature. It can be synthesized by reacting hydrocarbon gas with sulfur. It is an important raw material for preparing viscose rayon and cellophane film.
Application
Carbon disulfide has been used for the desorption of air samples during analysis of trihalomethanes (THMs) in those samples.
It may be used in the preparation of dibenzyl trithiocarbonate, a RAFT (Reversible Addition-Fragmentation Transfer) chain-transfer agent (CTA). It may be employed as a solvent in the preparation of benzyl chlorodithioformate.
Carbon disulfide may be used in the xanthogenation of cellulose and in the synthesis of poly(ethylene trithiocarbonate) and dithiocarbamates. C2S may undergo hydrolysis catalyzed by nanosized titania and zirconia to form CO2 and H2S. It can also generate 1,3-dithiolium carbenes via cycloaddition with acetylenes.
Signal word Danger
Hazard statements H225-H315-H319-H332-H361fd-H372
Precautionary statements P210-P260-P280-P305 + P351 + P338-P370 + P378-P403 + P235
RIDADR UN1131 – class 3 – PG 1 – Carbon disulfide
WGK Germany 2
RTECS FF6650000
Flash Point(F) -22°F
Flash Point(C) -30°C
Reviews (0)
Be the first to review “Carbon Disulfide 99.9%+, ACS Reagent, 2 Liter” Cancel reply
Shipping & Delivery











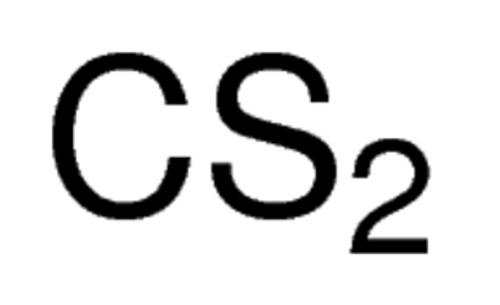










Reviews
There are no reviews yet.